| ● | 2015 | Analytical/ Formulation/ Bio Process Development/ Synthesis Lab Q4 2015 |
| ● | 2016 | Microbial Pilot Plant ,5L~300L Aseptic Injectable Pilot Plant , 4000 Vials/ Batch |
| ● | 2017 | Aseptic Injectable Plant , 40,000 Vials/ Batch Dedicated Oncology API & Lyophilization Vial Plant , 40,000 Vials/ Batch Peptide Pilot Plant |
| ● | 2018 | Cartridge Pen Injectable Line,2,000 Pen/Hour Pre-Filled Syringe Line ,>10,000 PFS/Hour |
| ● | 2019 | Microbial Commercial Plant, 3,000LX2 (Under Construction) Animal Cell Production Pilot Plant Animal Cell Production Commercial Plant (Monoclonal Antibody Drugs) |
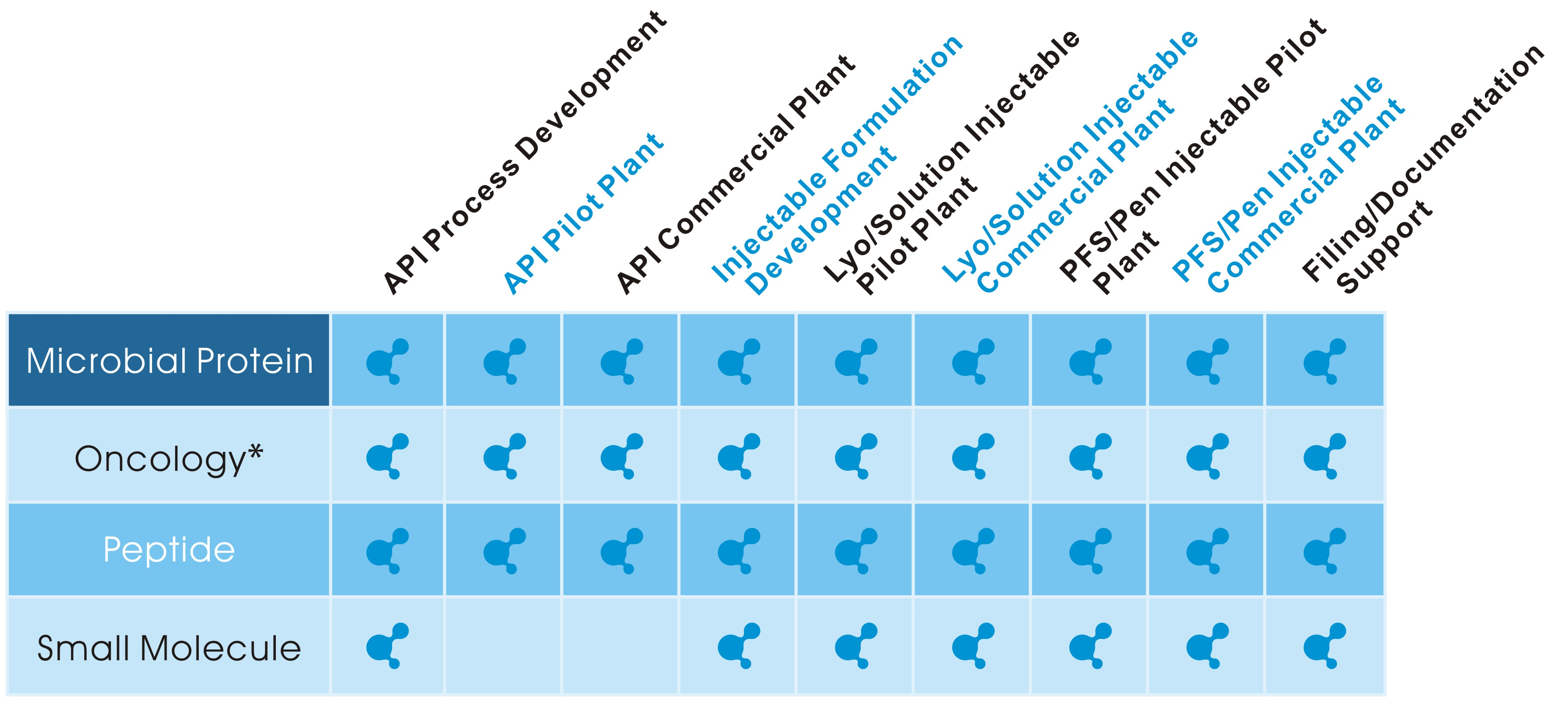
Our facilities are located in Northern and Southern of Taiwan, details could be found from below


